Abstract
Platelet (PLT) transfusion refractoriness (PTR) is defined as the recurrent failure to obtain a satisfactory response to PLT transfusions from random donors. Prior studies have shown that up to 50% of patients with underlying hematologic disorders and a history of more than 10 PLT transfusions develop PTR. Appropriate response to PLT transfusion is defined as PLT increase of more than 30% 1 hour post-transfusion and more than 20% 24 hours post transfusion (Rebulla. Transfus Med 1993). Immune-mediated etiologies of PTR consist of alloimmunization to human leucocyte antigen (HLA) and/or human platelet antigen (HPA), most commonly caused by prior antigen exposure from transfusions, transplantation or pregnancy. There are recent data suggesting that the destruction of PLT in PTR may be related to activation of the classical pathway of complement by HLA alloantibodies bound to transfused PLT (Vo et al. Blood 2016). In vitro data has shown that for the management of patients with PTR, HLA antibodies identified by a C1q-based assay are more clinically relevant than antibodies identified by the IgG binding-based method, as complement fixation, and not binding alone, is the essential step for PLT destruction (Fontaine, Transfusion 2011). Autoantibodies against PLT antigens have been shown to trigger activation of complement via the classical pathway resulting in the deposition of complement on the platelet surface. C1 esterase inhibitor (C1-INH) is one of the serine protease inhibitors and regulates the classical pathway of complement by blocking C1 esterase and is approved by FDA for prophylactic and therapeutic use in hereditary angioedema. (Zeerleder. Semin Thromb Hemost 2011, Zuraw et al. NEJM 2010) C1-INH has been reported to result in immediate platelet count improvement in patients with refractory immune thrombocytopenia (Roesch. Am J Hematol Oncol. 2016) and has also been successfully utilized to inhibit hemolysis after transfusion of antigen mismatched PRBCs (Warner. ASH 2016). Based on these data, we hypothesized that the prophylactic administration of a C1 would interrupt activation of the classical complement pathway and would overcome severe life threatening PTR by reducing the destruction of transfused PLTs in the _INH reticuloendothelial system
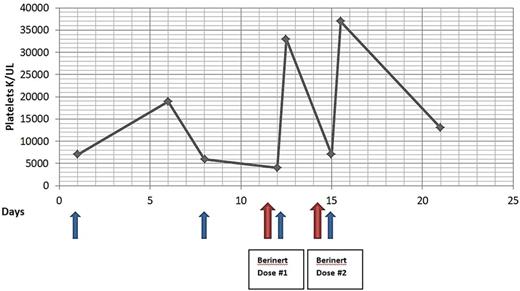
A 75yo female with a history of pre-B ALL in complete remission, developed treatment related AML. During therapy for AML she developed PTR. Her PLT recovery 24 hours after transfusion was consistently below 20% and her PLT count remained critically low (< 10 k/mcL). Further investigation with indirect PLT screen demonstrated plasma reactivity with 10 of 10 HLA-typed PLT in the Solid Phase Adherence Assay. Given the critical clinical circumstances, it was decided to administer the commercially available C1-INH, Berinert®, prior to transfusion of HLA mismatched PLT. After the first dose of 2000u C1-INH and transfusion of HLA mismatched PLT the 1 hour post transfusion PLT count increased to 33 k/mcL from baseline of 4 k/mcL. A second dose of C1-INH was given before administering the next PLT transfusion for a PLT count of 7 k/mcL and the 1 hour post transfusion PLT count was 37 k/mcL. This treatment strategy was continued for 2 months. The patient received a total of 13 doses of C1-INH. The mean PLT count nadir prior to and after initiation of C1-INH was 7 k/mcL and 23 k/mcL, respectively.
We present the first reported case utilizing prophylactic inhibition of complement with C1-INH prior to PLT transfusion in a highly alloimmunized patient. With this strategy, the patient achieved clinically significant improvement in PLT response post transfusions. Furthermore, the mean of the nadir PLT counts while on C1-INH were higher. These two findings are clinically important as studies have shown significant reduction in spontaneous bleeding risk with PLT above 10 k/mcL. We hypothesize that inhibiting the activation of complement via the classical pathway could potentially be effective in prevention of the destruction of transfused PLTs in patients who are PTR secondary to alloimmunization. Future directions would include clinical trials exploring complement inhibition's role in treating PTR and possibly preventing alloantibody formation in heavily transfused patients.
Broome: Alexion Pharmaceuticals: Honoraria; TrueNorth Therapuetics: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.